Featured
- Get link
- X
- Other Apps
Calculate Activation Energy Of Reverse Reaction
Calculate Activation Energy Of Reverse Reaction. The activation energy units are lcal/mo, kj/mol, and j/mol. Note that the enthalphy change is negative for.
If we know the rate constant k1 and k2 at t1 and t2 the activation energy formula is. What is the activation energy of the reverse reaction chegg, keeping this in mind? R = ideal gas constant=8.3145 j/k·mol.
As Activation Energy Term E A Increases, The Rate Constant K Decreases And Therefore The Rate Of Reaction Decreases.
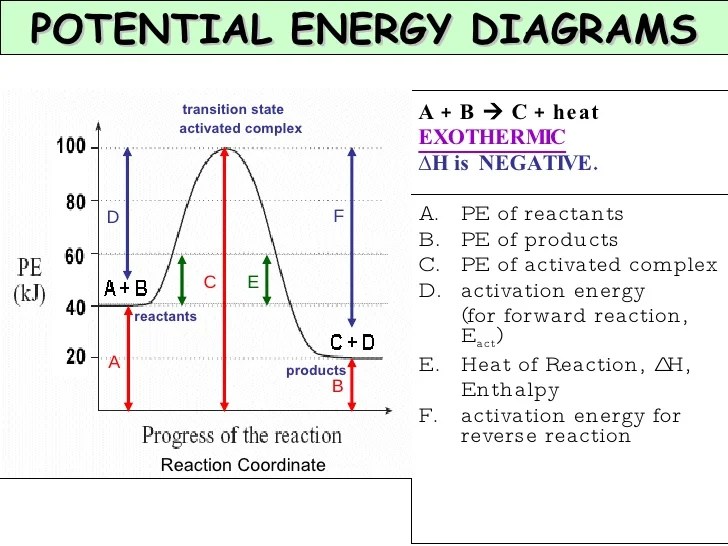
The activation energy of the reverse reaction by this diagram is given by e. It makes sense that the reverse activation energy for an exothermic reaction would be ∆h + ea since those values added together gives you the height of the hump if you imagine the graph mirrored or going backwards. Calculate the activation energy of the reaction.
The Arrhenius Equation Allows Us To Calculate Activation Energies If The Rate Constant Is Known, Or Vice Versa.
When you draw a reaction profile for an endothermic reaction vs. The activation energy of the reverse reaction is just the difference in energy between the product(s) (right) and the transition state (hill). The activation energy for the forward reaction.
The Activation Energy Units Are Lcal/Mo, Kj/Mol, And J/Mol.
T 1 and t 2 = absolute temperatures (kelvin) k 1 and k 2 = the reaction rate constants at t 1 and t 2. The curve’s peak represents the transition state. Note that the enthalphy change is negative for.
We Can Use The Arrhenius Equation To Relate The Activation Energy And The Rate Constant, K, Of A Given Reaction:
2.5 5000 e4 ks 42.0 트 at 20.0°c, milk sours in about 64 hours; In order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature (energy) of the system. To find the energy change in the opposite direction, you would add the energy given out by the system in addition to the existing activation energy.
R = The Ideal Gas Constant = 8.3145 J/K·mol.
This is asking you to draw a potential energy diagram for an endothermic reaction. I do know that the activation energy for the reverse reaction is larger than. Recall that \(\delta h_{\mathrm{rxn}}\), the enthalpy of reaction, is positive for endothermic reactions, i.e.
Popular Posts
Soft Tissue Injury Compensation Calculator
- Get link
- X
- Other Apps
Comments
Post a Comment